フィニッシャーの有効性についての文献 Effectiveness of supplementary irrigant agitation with the Finisher GF Brush on the debridement of oval root canals instrumented with the Gentlefile or nickel titanium rotary instruments
1Discipline of Endodontology, Faculty of Dentistry, The University of Hong Kong, Hong Kong; 2Centralized Research Laboratories, Faculty of Dentistry, The University of Hong Kong, Hong Kong, Hong Kong SAR; 3Department of Conservative Dentistry and Endodontics, Nair Hospital Dental College, Mumbai, India; and 4Department of Operative Dentistry and Endodontics, Guanghua College of Stomatology, Sun Yat-sen University, Guangzhou, China
Abstract
Neelakantan P, Khan K, Li KY, Shetty H, Xi W. Effectiveness of supplementary irrigant agitation with the Finisher GF Brush on the debridement of oval root canals instrumented with the Gentlefile or nickel titanium rotary instruments. International Endodontic Journal.
Aim To examine the efficacy of a novel supplementary irrigant agitating brush (Finisher GF Brush, MedicNRG, Kibbutz Afikim, Israel) on the debridement of root canals prepared with a novel stainless steel rotary instrumentation system (Gentlefile; MedicNRG), or nickel titanium rotary instruments in oval root canals.
Methodology Mandibular premolars (n = 72) were selected and divided randomly into three experimental groups (n = 24) after microCT scanning: group 1, canal preparation to rotary NiTi size 20, .04 taper (R20); group 2, rotary NiTi to size 25, .04 taper (R25) and group 3, Gentlefile size 23, .04 taper (GF). Specimens were subdivided into two subgroups: subgroup A, syringe-and-needle irrigation (SNI); subgroup B, Finisher GF Brush (GB). Ten untreated canals served as controls. Specimens were processed for histological evaluation, and the remaining pulp tissue (RPT) was measured. Data were analysed using Mann–Whitney and Kruskal–Wallis tests (P = 0.05).
Results All experimental groups had significantly less RPT than the control (P < 0.05). Group 3B (GFGB) had significantly less RPT than groups 1B (R20- GB) and 2B (R25-GF; P < 0.05). When irrigated with SNI, there was no significant difference in the RPT between the three groups (P > 0.05). When instrumented with R20, there was no significant difference between SNI and GF (P < 0.05) whilst GB had significantly less RPT than SNI for R25 (P < 0.05).
Conclusions Supplementary irrigant agitation with the Finisher GF Brush improved the debridement of canals prepared with Gentlefile and size 25, .04 taper rotary NiTi. Root canal debridement did not significantly differ between the instruments when syringe irrigation was used.
Keywords: debridement, nickel titanium, pulp tissue, rotary, stainless steel.
Received 3 November 2017; accepted 18 January 2018
Correspondence: P. Neelakantan, Discipline of Endodontology, Faculty of Dentistry, The Prince Philip Dental Hospital, The University of Hong Kong, 34, Hospital Road, Sai Ying Pun, Hong Kong SAR (Tel.: +85228590581; fax: +85225599013; e-mail: prasanna@hku.hk).
Introduction
The process of chemomechanical preparation of the root canal system involves removal of infected soft and hard tissue, microbial biofilms and creating a shape that allows effective filling (Siqueira et al. 2017). To accomplish this goal, instruments made of stainless steel (SS) or nickel-titanium (NiTi) are used in conjunction with irrigating solutions (Peters et al. 2016). The introduction of greater tapered instruments used in rotary or reciprocating motion has helped shape root canals more rapidly, but not necessarily made them cleaner (Busquim et al. 2015, Neves et al. 2016, Siqueira et al. 2017). In part, this is attributed to the inability of these instruments to contact the entire canal surface (Peters et al. 2001, Paque et al. 2009, Siqueira et al. 2017). This results in uninstrumented areas, leaving behind persistent microbes, that may result in post-treatment apical periodontitis (Siqueira et al. 2017).
The advantage of NiTi instruments is their superelasticity whilst their disadvantage is unexpected fracture because of fatigue (cyclic or torsional; Parashos & Messer 2006, Nguyen et al. 2014, Pedulla et al. 2017). On the other hand, SS instruments are not considered as flexible as NiTi but undergo deformation prior to fracture (Pruett et al. 1997, Neelakantan et al. 2016a,b). A substantial number of reports exist on the association of rotary and reciprocating NiTi instruments with cracks in roots (De-Deus et al. 2014, 2017, Capar et al. 2015); although there is no clear evidence, this possibility has not been conclusively over-ruled.
Gentlefile (MedicNRG, Kibbutz Afikim, Israel) is a novel rotary instrumentation system fabricated using SS (Moreinos et al. 2016). The files of this system consist of a central braided cable of diameter <0.15 mm in the apical third, on which a second wire with diameter <0.20 mm is coiled. Towards the middle and coronal portions of the instrument, a third wire <0.35 mm in diameter is coiled over the second wire. All files have a constant taper of 4% and an inactive passive tip. The tip diameters are 0.21, 0.23, 0.26, 0.29 and 0.34 mm, which do not conform to the ISO diameters of endodontic instruments (Moreinos et al. 2016). The main difference between these instruments and rotary NiTi instruments is that they do not cut into dentine; rather, they abrade/scrape the dentinal walls. An automated, non-customizable handpiece is used to operate the instruments at 6500 rpm.
Irrigation of the root canal system using a needle and syringe does not generate sufficient hydrodynamic shear stresses to dislodge the biofilms or tissue that adhere to the root canal walls (Goode et al. 2013, Chen et al. 2014, Neelakantan et al. 2016a,b). To counteract this disadvantage, irrigant agitation strategies have been suggested (Plotino et al. 2016). The Gentlefile system also offers a brush (Finisher GF Brush) which consists of six strands of SS that open outwards automatically when operated in a handpiece at 6500 rpm (http://www.gentlefile.com/wp-content/uploads/2016/07/the-finisher-GF-brush-kit-6.7.pdf).
Contemporary preparation strategies using rotary or reciprocating instruments may not sufficiently plane the root canal walls, leaving behind remnant tissue and microbial biomass (De-Deus et al. 2011) which can result in re-infection or persistent infection (Siqueira 2001, De-Deus et al. 2011, Zhang et al. 2015, Neelakantan et al. 2016a,b). This is specifically true in the case of oval canals, which are challenging to clean and shape due to the circular shape of preparation achieved by instruments (De-Deus et al. 2011, Busquim et al. 2015). To optimize preparation of complex canal anatomies, modified instrumentation systems such as self-adjusting file (SAF; ReDent-Nova, Ra’anana, Israel) and Gentlefile have been introduced. There is evidence that root canal preparation with the SAF system results in a 57% reduction in pulp tissue, compared with a rotary instrumentation system (ProTaper; Dentsply Sirona Endodontics, York, PA, USA) in oval canals. In this afore-mentioned study, the SAF left behind only 9.3 ± 3.7% pulp tissue in oval canals (De-Deus et al. 2011). Surprisingly, only one study has been published on the Gentlefile system (Moreinos et al. 2016), and the efficacy of Gentlefile in root canal debridement, compared with rotary NiTi instruments, has not been evaluated thus far; there were no reports on root canal debridement when using the Finisher GF Brush.
A recent report demonstrated that even after root canal preparation to a size 25 or 40, 20% of the root canal surface area of instrumented mesiolingual canals of mandibular molars had remnant pulp tissue and approximately 35% of the canal wall of premolars remained untouched, respectively (Siqueira et al. 2017). It is also known that 30–60% of root canals harbour microbes after root canal preparation. Remnant tissues could harbour bacteria or serve as a source of nutrition for bacteria that are not removed from the root canals resulting in persistent or secondary infection (De-Deus et al. 2011, Neelakantan et al. 2016a,b, Siqueira et al. 2017).
Therefore, this study was performed to examine the effectiveness of supplementary irrigant agitation with the Finisher GF Brush, in debriding oval root canals in extracted teeth, after preparation with Gentlefile or rotary NiTi instruments. The null hypotheses were that supplementary irrigant agitation did not significantly reduce the amount of pulp tissue compared with syringe irrigation and that there was no significant difference between the experimental groups in the percentage of remaining pulp tissue (RPT).
Materials and methods
Selection of teeth
From a collection of recently extracted non-carious human mandibular first premolars, 82 specimens were chosen based on a pilot study, to detect differences with a statistical power of 80%. The protocol was approved by the Institutional Review Board and Ethics Committee (EC-79/CONS-08ND/2017). Only teeth with normal pulps, as determined by sensibility testing using the cold test (Green Endo Ice; Hygenic Corp., Akron, OH, USA) and an electric pulp tester (Kerr Analytic Technology Corp., Redmond, WA, USA), prior to the extraction, were included. These teeth were extracted as part of an orthodontic treatment plan and were unrelated to the present experiment. Informed consent was obtained from the patients. The patients were within the same age group (20–25 years), and hence, the size of the teeth and pulp chamber, as well as the amount of tissue within the root canal system, was likely to be similar. The soft tissues attached to the external surface of the teeth were removed using a curette, following which the specimens were kept in individual vials containing 5 mL of 10% formalin until use. The validity of the experimental design has been demonstrated previously (De-Deus et al. 2011, Neelakantan et al. 2016a,b).
The 82 specimens were chosen following the micro-computed tomographic scanning (SkyScan 1172; Bruker microCT, Kontich, Belgium) to confirm the presence of a single oval canal (mesiodistal diameter 2.5 times larger than the buccolingual diameter, about 5 mm coronal to the apex; De-Deus et al. 2011). Ten of the 82 specimens served as histological controls. A conventional occlusal access cavity was performed with bur no. 856 (Komet Dental GmBH, Lemgo, Germany) in a high-speed handpiece with water cooling. A size 10 stainless steel K-file (Mani Inc., Togichi, Japan) was inserted into the root canals until the tip was just visible at the apical foramen. Working length was defined as 1 mm short of this length.
Root canal preparation
Specimens (n = 72) were randomly allotted to one of the three groups (n = 24) with the aid of a computer algorithm (http://www.random.org):
Group 1: Rotary instrumentation (EdgeFile X7; EdgeEndo, Albuquerque, New Mexico, USA) to size 20, .04 taper (R20);
Group 2: Rotary instrumentation (EdgeEndoX7) to size 25, .04 taper (R25); and
Group 3: Rotary Instrumentation to Gentlefile size 23 (GF; Fig. 1a).
Instrumentation of the canals was performed in a closed apical system. The number of instruments used in sequence for the groups was different. Whilst only one instrument was used in group 1 (size 20, .04 taper), group 2 used two instruments in sequence (size 20, .04 taper and size 25, .04 taper). In group 3, the orifice opener (grey instrument; size 0.22) was used first followed by the red instrument (size 0.23). A new instrument was used for every specimen and the instrumentation technique followed the manufacturers’ instructions (http://www.gentlefile.com/wp-content/uploads/2015/05/Protocol-of-use.pdf).
As the number of instruments was different across the groups, standardization was achieved in terms of time of instrumentation as well as irrigation. The volume and time of irrigation of 3% sodium hypochlorite (NaOCl) during instrumentation was standardized to 6 mL over a 3-min period in all the specimens. In groups 2 and 3, 2 mL of NaOCl was used during instrumentation and 2 mL of NaOCl was used between the two instruments. In group 1, 4 mL of NaOCl was used during instrumentation and 2 mL was used after instrumentation was completed. During irrigation, the needle (31G side-vented needle, NaviTip; Ultradent Products Inc., South Jordan, UT, USA) was placed passively into the canal, 1 mm from the apical foramen, without binding. A size 10 K-file (Mani Inc.) was used to maintain apical patency.
Following the completion of instrumentation, specimens in each group were randomly allocated to one of the subgroups (n = 12):
Subgroup A, syringe-and-needle irrigation (SNI) using a 31G side-vented needle (Ultradent Products Inc.) placed passively into the canal, 1 mm short of the working length;
Subgroup B, Finisher GF Brush (GB; Fig. 1b) agitation placed 1 mm short of the working length, used in an up-and-down motion according to the manufacturer’s instructions.
The volume of irrigant (3% NaOCl) and duration of agitation were standardized to 2 mL and 2 min, respectively. Canals were then irrigated with 3 mL of 17% EDTA followed by 3 mL distilled water to remove the EDTA. The root canals were then dried with absorbent paper points (Dentsply Sirona Endodontics). All experimental procedures were performed by one experienced endodontist.
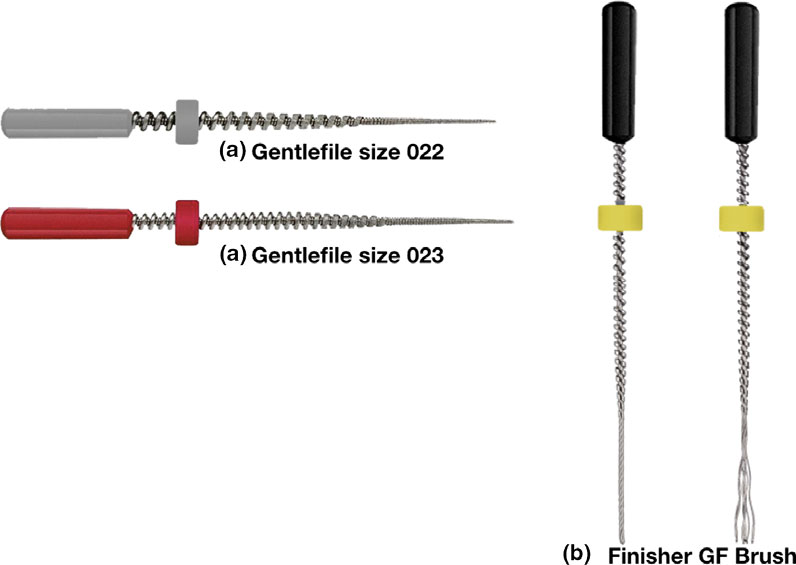
Figure 1 (a) Gentlefile instruments size 022 and 023: The instruments are composed of layers of stainless steel wires wrapped on a cable core. (b) Finisher GF Brush: This instrument is composed of six strands of SS wires that open outwards when operated at 6500 rpm.
Histologic evaluation
After the endodontic procedure, the specimens were processed for histological examination by fixing in 10% buffered formalin for 48 h, washed in water and demineralized in a solution of 10 wt% hydrochloric acid and 5 wt% EDTA for 1–2 weeks. The specimens were then rinsed with water, dehydrated and with the end of demineralization being confirmed radiographically. The teeth were then rinsed in tap water for 24 h, sectioned to achieve 6 μm-thick serial sections from the root canal. The sections were mounted on glass slides and stained with haematoxylin–eosin. Twenty serial sections were chosen from each specimen and visualized using a digital microscope (Nikon Eclipse LV100POL; Nikon Instruments Inc., Kawasaki, Japan) with a digital camera (Nikon DS-Ri1; Nikon Instruments Inc.) at 2× and 5× magnification.
Captured images were processed and analysed in an image analysis software (NIS Elements AR 3.10; Nikon Instruments Inc.). The area percentage of RPT in the root canals was determined based on a previously documented method (De-Deus et al. 2011, Neelakantan et al. 2016a,b). Briefly, the cross-sectional area of the root canal and RPT were measured to calculate the RPT. Evaluations were performed by an operator who was blinded to the experimental groups.
Statistical analysis
The data were evaluated for normality using Shapiro–Wilk test. As the data did not follow normal distribution, nonparametric tests (Mann–Whitney U test and Kruskal–Wallis test) were used. To adjust for the multiple testing, the significance level for comparisons amongst three groups (one-way ANOVA) or Kruskal–Wallis test) was set at P < 0.025 whilst the significance level for comparisons between two groups and pairwise comparison was set at P = 0.05.
Results
The percentage of RPT in the experimental groups and untreated control appears in Table 1. All experimental groups were associated with significantly less RPT than the control (Mann–Whitney test; P < 0.05).
When syringe-and-needle irrigation was employed, there was no significant difference in the RPT between the three groups (Kruskal–Wallis test; P > 0.05; Fig. 1). Whilst group 1 (R20-SNI) had substantial RPT on all the walls of the root canal in the examined sections, group 2 (R25-SNI) demonstrated tissue remnants along with debris in the eccentricities of the oval canal (Fig. 1). A substantial amount of predentine was also observed in the R20 specimens (data not analysed). However, when the GB (subgroup B) was used, there was a significant difference between the three groups, with group 3B (GF-GB) having the least RPT (Kruskal–Wallis test; P < 0.05; Fig. 2). There was no significant difference in the RPT between subgroups A and B in group 1 (R20; Mann–Whitney test; P < 0.05), whilst subgroup B (GB) had significantly less RPT than subgroup A (SNI) in groups 2 (R25) and 3 (GF; Mann–Whitney test; P < 0.05; Fig. 3).
Table 1 Means ± standard deviations (SD) and medians (minimum–maximum) of the percentage of RPTs (%) in the experimental and control groups
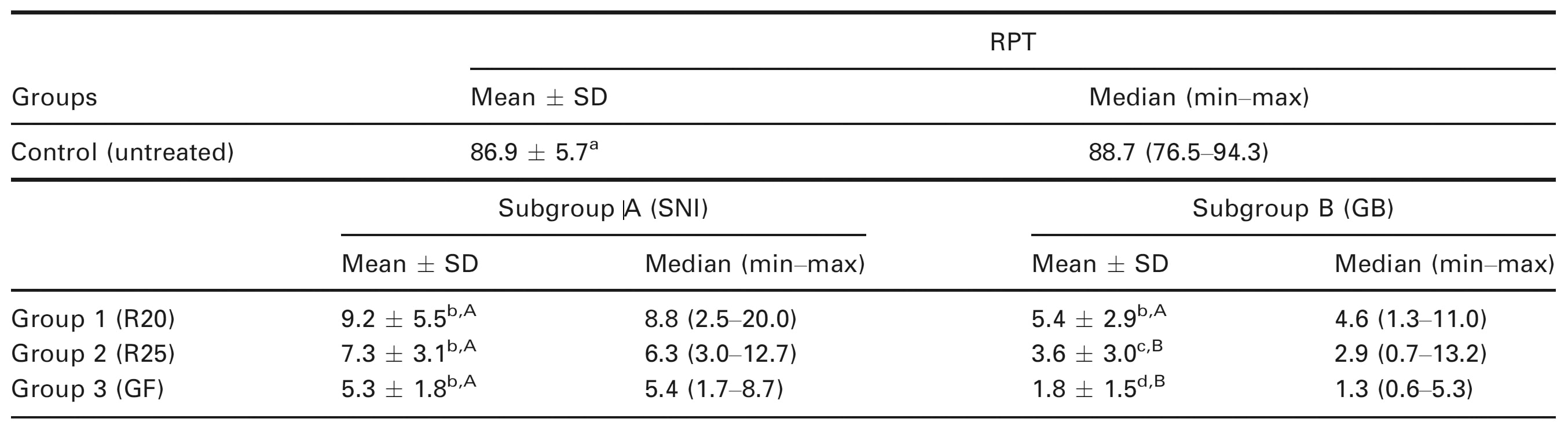
For each column, mean values that share the same superscript lower-case letter were not significantly different at the 5% level (Control versus experimental groups in subgroup A, and Control versus experimental groups in subgroup B). For each row, mean values that share the same superscript upper-case letter were not significantly different at the 5% level.
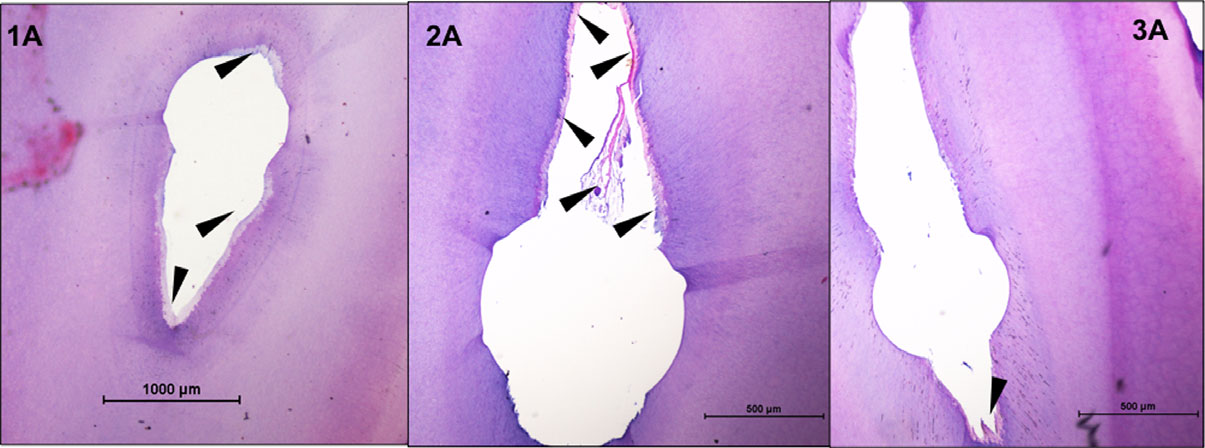
Figure 2 Representative sections from root canals (4 mm from the apex) in teeth prepared with rotary NiTi instruments (groups 1A and 2A) and Gentlefile (group 3A) and syringe-and-needle irrigation. Black arrows point to the predentine and remnant pulp tissue.
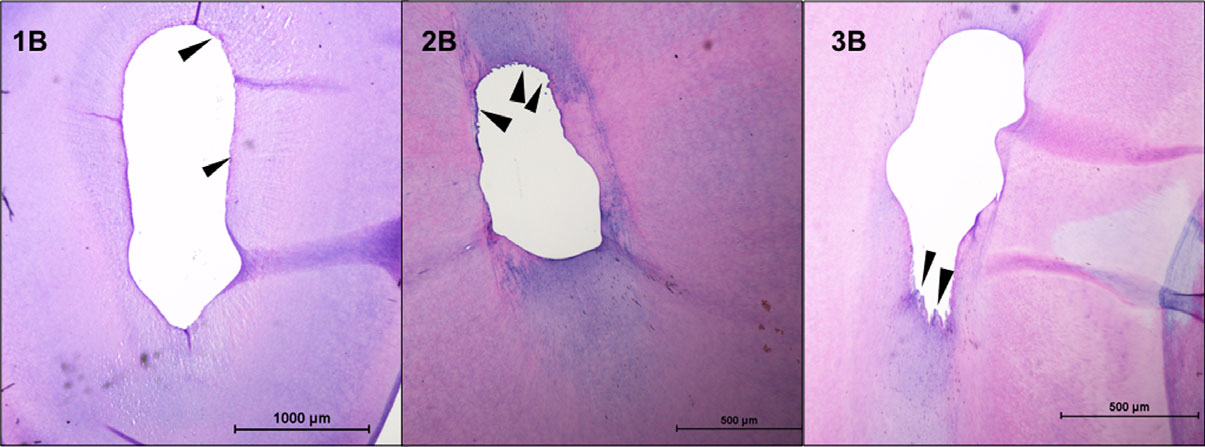
Figure 3 Representative sections from root canals (4 mm from the apex) in teeth prepared with rotary NiTi instruments (groups 1B and 2B) and Gentlefile (group 3B) and Finisher GF Brush irrigant agitation. Black arrows point to the remnant pulp tissue.
Discussion
This study evaluated the effectiveness of irrigant agitation with the Finisher GF Brush after root canal preparation with the Gentlefile or NiTi instruments. The rationale behind choosing two rotary NiTi instrument sizes (sizes 20 and 25) was that the GF is offered in non-standardized sizes and the size 23 was used in this study. Hence, the debridement efficacy of one instrument smaller and one larger, compared with the GF was evaluated. All instruments had a 4% taper. Understandably, mechanical instrumentation alone can remove a certain amount of pulp tissue from the root canal system. Thus, potentially the size 25 instrument could remove more pulp tissue than the size 20 rotary NiTi instrument. However, the study design had to strike a balance between standardization and clinical relevance. Thus, all efforts were made to standardize the instrumentation and irrigation time to minimize the possible effects of number of instruments.
The use of the Finisher GF Brush resulted in significantly less RPT in groups 2 and 3, when compared to syringe irrigation. So, the null hypotheses can be partially accepted. The results of this study revealed that when root canals were prepared with rotary NiTi instruments, those prepared to a size 25, .04 taper had less pulp tissue than those prepared to size 20, .04 taper when a syringe and needle was used for irrigation, although this difference was not statistically significant. The location of the remnant tissues was an interesting finding; specimens in the R20-SNI group consistently had pulp remnants along the entire perimeter of the canal whilst those in the R25-SNI group had tissue and debris packed in the eccentricities. Similarly, root canals instrumented with GB and irrigated with a syringe and needle did not have significantly lesser RPT than groups instrumented with rotary NiTi. The specimens in this group did not have a ‘characteristic’ pattern in the presence of remnant tissue as could be noted in groups 1A and 2A. Hence, it may be that the GF was able to touch more of the canal wall than rotary NiTi instruments, but the syringe irrigation was unable to dislodge the tissues. The finding that syringe irrigation does not result in clean canals is not new and corroborates with the findings of several other studies (Jiang et al. 2012, Chen et al. 2016, Neelakantan et al. 2016a,b, Mohmmed et al. 2017), further validating the study design.
This study used relatively smaller sizes of root canal preparation. No effort was made to categorize specimens based on the initial apical diameter to reflect the clinical scenario. It was previously reported that a size 35, .04 taper preparation was needed to produce root canals with clean dentinal walls (Khademi et al. 2006) using scanning electron microscopic analysis. Although limited by the extent of apical preparation diameters evaluated in this study, the results indicate that there is no significant impact of apical preparation sizes in reducing remnant tissue, when syringe irrigation is employed. Furthermore, the results of this study reveal that it may be possible to achieve root canal cleaning with smaller apical sizes, in singlerooted teeth, when specific conditions of instrumentation and irrigant activation are used. This may consequently challenge the paradigm that dentine needs to be removed to obtain clean canals. Studies comparing several apical preparation diameters and tapers are in progress. Furthermore, studies need to be performed to evaluate the effect of such preparation sizes on biofilm disruption within the root canal and dentinal tubules.
In general, it was observed that specimens instrumented with GF had less RPT than the control and other experimental groups, albeit this difference was not significant. It was reported that the SAF instrument, which is designed like a metal mesh, generates sonic activation of irritant when used within root canals as it vibrates at 5000 vibrations min-1 (Metzger et al. 2010, De-Deus et al. 2011). Such a hypothesis is plausible for the GF instrument as well, as it rotates at 6500 rpm. This high rotational speed could also generate sufficient centrifugal forces to drive irrigants into the eccentricities of the oval canal (Garip et al. 2010), thereby resulting in significantly cleaner canals. When the Finisher GF Brush was used for irrigant agitation, there were significant differences in RPT between the three groups. This demonstrates that irrigant agitation strategy plays a more important role than the apical size of preparation in debriding root canals. The Finisher GF Brush works by opening the six SS strands when rotated. This may scrape the canal walls to remove tissue and microbial biofilms that are attached more effectively than syringe irrigation (De-Deus et al. 2011). This is only the first reported work on the Finisher GF Brush, and future studies should compare this approach with other strategies such as ultrasonic and sonic activated irrigation.
Conclusion
The least amount of remnant pulp tissue was found in root canals instrumented with the Gentlefile followed by supplementary Finisher GF Brush usage. When root canals were prepared to the larger size (i.e. 25), supplementary irrigant agitation with the Finisher GF Brush reduced the remnant tissue significantly more than syringe irrigation.
Acknowledgements
The authors sincerely thank Dr. Obadah Austah, University of Texas Health Science Centre at San Antonio, for his critical review of this manuscript. We thank MedicNRG for providing the instruments for this study.
Conflict of interest
Dr Neelakantan reports that Gentlefile instruments and Finisher GF Brush were donated by the company. All other authors have stated explicitly that there are no conflict of interests in connection with this article.
References
- Busquim S, Cunha RS, Freire L, Gavini G, Machado ME, Santos M (2015) A micro-computed tomography evaluation of long-oval canal preparation using reciprocating or rotary systems. International Endodontic Journal 48, 1001–6.
- Capar ID, Uysal B, Ok E, Arslan H (2015) Effect of the size of the apical enlargement with rotary instruments, single-cone filling, post space preparation with drills, fiber post removal, and root canal filling removal on apical crack initiation and propagation. Journal of Endodontics 41, 253–6.
- Chen JE, Nurbakhsh B, Layton G, Bussmann M, Kishen A (2014) Irrigation dynamics associated with positive pressure, apical negative pressure and passive ultrasonic irrigations: a computational fluid dynamics analysis. Australian Endodontic Journal 40, 54–60.
- Chen S, Liu J, Dong G et al. (2016) Comparison between ultrasonic irrigation and syringe irrigation in clinical and laboratory studies. Journal of Oral Science 58, 373–8.
- De-Deus G, Souza EM, Barino B et al. (2011) The self-adjusting file optimizes debridement quality in oval-shaped root canals. Journal of Endodontics 37, 701–5.
- De-Deus G, Silva EJ, Marins J et al. (2014) Lack of causal relationship between dentinal microcracks and root canal preparation with reciprocation systems. Journal of Endodontics 40, 1447–50.
- De-Deus G, Cesar de Azevedo Carvalhal J, Belladonna FG et al. (2017) Dentinal microcrack development after canal preparation: a longitudinal in situ micro-computed tomography study using a cadaver model. Journal of Endodontics 43, 1553–8.
- Garip Y, Sazak H, Gunday M, Hatipoglu S (2010) Evaluation of smear layer removal after use of a canal brush: an SEM study. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 110, e62–6.
- Goode N, Khan S, Eid AA et al. (2013) Wall shear stress effects of different endodontic irrigation techniques and systems. Journal of Dentistry 41, 636–41.
- Jiang LM, Lak B, Eijsvogels LM, Wesselink P, van der Sluis LW (2012) Comparison of the cleaning efficacy of different final irrigation techniques. Journal of Endodontics 38, 838–41.
- Khademi A, Yazdizadeh M, Feizianfard M (2006) Determination of the minimum instrumentation size for penetration of irrigants to the apical third of root canal systems. Journal of Endodontics 32, 417–20.
- Metzger Z, Teperovich E, Cohen R, Zary R, Paque F, Hulsmann M (2010) The self-adjusting file (SAF). Part 3: removal of debris and smear layer-A scanning electron microscope study. Journal of Endodontics 36, 697–702.
- Mohmmed SA, Vianna ME, Penny MR, Hilton ST, Mordan N, Knowles JC (2017) Confocal laser scanning, scanning electron, and transmission electron microscopy investigation of Enterococcus faecalis biofilm degradation using passive and active sodium hypochlorite irrigation within a simulated root canal model. MicrobiologyOpen 6, e00455. https://doi.org/10.1002/mbo3.455.
- Moreinos D, Dakar A, Stone NJ, Moshonov J (2016) Evaluation of time to fracture and vertical forces applied by a novel Gentlefile system for root canal preparation in simulated root canals. Journal of Endodontics 42, 505–8.
- Neelakantan P, Devaraj S, Jagannathan N (2016a) Histologic assessment of debridement of the root canal isthmus of mandibular molars by irrigant activation techniques ex vivo. Journal of Endodontics 42, 1268–72.
- Neelakantan P, Reddy P, Gutmann JL (2016b) Cyclic fatigue of two different single files with varying kinematics in a simulated double-curved canal. Journal of Investigative and Clinical Dentistry 7, 272–7.
- Neves MA, Provenzano JC, Rocas IN, Siqueira JF Jr (2016) Clinical antibacterial effectiveness of root canal preparation with reciprocating single-instrument or continuously rotating multi-instrument systems. Journal of Endodontics 42, 25–9.
- Nguyen HH, Fong H, Paranjpe A, Flake NM, Johnson JD, Peters OA (2014) Evaluation of the resistance to cyclic fatigue among ProTaper Next, ProTaper Universal, and Vortex Blue rotary instruments. Journal of Endodontics 40, 1190–3.
- Paque F, Ganahl D, Peters OA (2009) Effects of root canal preparation on apical geometry assessed by microcomputed tomography. Journal of Endodontics 35, 1056–9.
- Parashos P, Messer HH (2006) Rotary NiTi instrument fracture and its consequences. Journal of Endodontics 32, 1031–43.
- Pedulla E, Lizio A, Scibilia M et al. (2017) Cyclic fatigue resistance of two nickel-titanium rotary instruments in interrupted rotation. International Endodontic Journal 50, 194–201.
- Peters OA, Schonenberger K, Laib A (2001) Effects of four Ni-Ti preparation techniques on root canal geometry assessed by micro computed tomography. International Endodontic Journal 34, 221–30.
- Peters OA, Peters CI, Basrani B (2016) Cleaning and shaping the root canal system. In: Hargreaves KM, Berman LH, eds. Cohen’s Pathways of the Pulp, 11th edn. St Louis: Elsevier, pp 209–79.
- Plotino G, Cortese T, Grande NM et al. (2016) New technologies to improve root canal disinfection. Brazilian Dental Journal 27, 3–8.
- Pruett JP, Clement DJ, Carnes DL Jr (1997) Cyclic fatigue testing of nickel-titanium endodontic instruments. Journal of Endodontics 23, 77–85.
- Siqueira JF Jr (2001) Aetiology of root canal treatment failure: why well-treated teeth can fail. International Endodontic Journal 34, 1–10.
- Siqueira JF Jr, Perez AR, Marceliano-Alves MF et al. (2017) What happens to unprepared root canal walls: a correlative analysis using micro-computed tomography and histology/ scanning electron microscopy. International Endodontic Journal https://doi.org/10.1111/iej.12753.
- Zhang C, Du J, Peng Z (2015) Correlation between Enterococcus faecalis and persistent intraradicular infection compared with primary intraradicular infection: a systematic review. Journal of Endodontics 41, 1207–13.
